The pharmaceutical supply chain is an intricate, highly regulated system that ensures life-saving medicines reach patients on time and in full compliance.
Because drug supply chains are so complex and sensitive, even a small disruption, whether from geopolitical events, material shortages, or regulatory delays, can trigger drug shortages, stockouts, and price fluctuations across the healthcare system. For today’s pharma leaders, efficient supply chain operations are no longer enough. Success depends on adhering to strict quality standards, precision, visibility, and strong collaboration to build a resilient, future-ready network.
Key Takeaways:
- The pharmaceutical supply chain links every step from raw materials to patient delivery, governed by strict GMP and GDP standards to ensure safety and consistency.
- Even small disruptions—like material shortages or regional delays—can cause shortages and price shifts across healthcare systems.
- Data-driven visibility, AI forecasting, and RFID tracking improve accuracy, reduce waste, and prevent counterfeit infiltration.
- Non-porous plastic pallets support cold chain stability, automation, and hygiene, ensuring regulatory compliance and product integrity.
- Pooling plastic pallets through providers such as iGPS lowers maintenance costs, cuts waste, and improves traceability.
What Is the Pharmaceutical Supply Chain
The pharmaceutical supply chain connects every stage of a medicine’s journey from sourcing active pharmaceutical ingredients (APIs) and raw materials, to pharmaceutical manufacturing, packaging, distribution, and delivery to pharmacies and healthcare providers.
Because this system directly affects patient safety, it operates under strict Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) standards. Even minor supply chain disruptions can create a ripple effect that leads to drug shortages, pricing volatility, and compromised product quality. Every phase must ensure quality control, documentation accuracy, and traceability.
How Does the Pharmaceutical Supply Chain Work?
The pharmaceutical supply chain functions as a collaborative, data-driven ecosystem connecting manufacturers, distributors, and healthcare providers. Each stakeholder plays a critical role in ensuring medicines are produced, handled, and delivered safely.
This process depends on the coordination of manufacturers to maintain GMP quality standards, logistics teams to handle compliance and transport, and Pharmacy Benefit Managers (PBMs) to manage pricing and patient access.
Pharmaceutical Supply Chain Strategies for Resilience and Efficiency
Building a resilient supply chain means adopting proactive, data-backed strategies that enhance adaptability and reduce the risk of disruption.
Leading pharmaceutical manufacturers are:
- Diversifying suppliers and production sites to minimize geographic concentration and geopolitical risk.
- Investing in domestic manufacturing to secure supply of critical prescription drugs and generic medicines.
- Using advanced manufacturing technologies to improve efficiency and resource management.
- Implementing AI-driven inventory management systems to optimize stock levels and reduce waste.
- Collaborating across the supply chain network to improve transparency, reduce costs, and align with global quality standards.
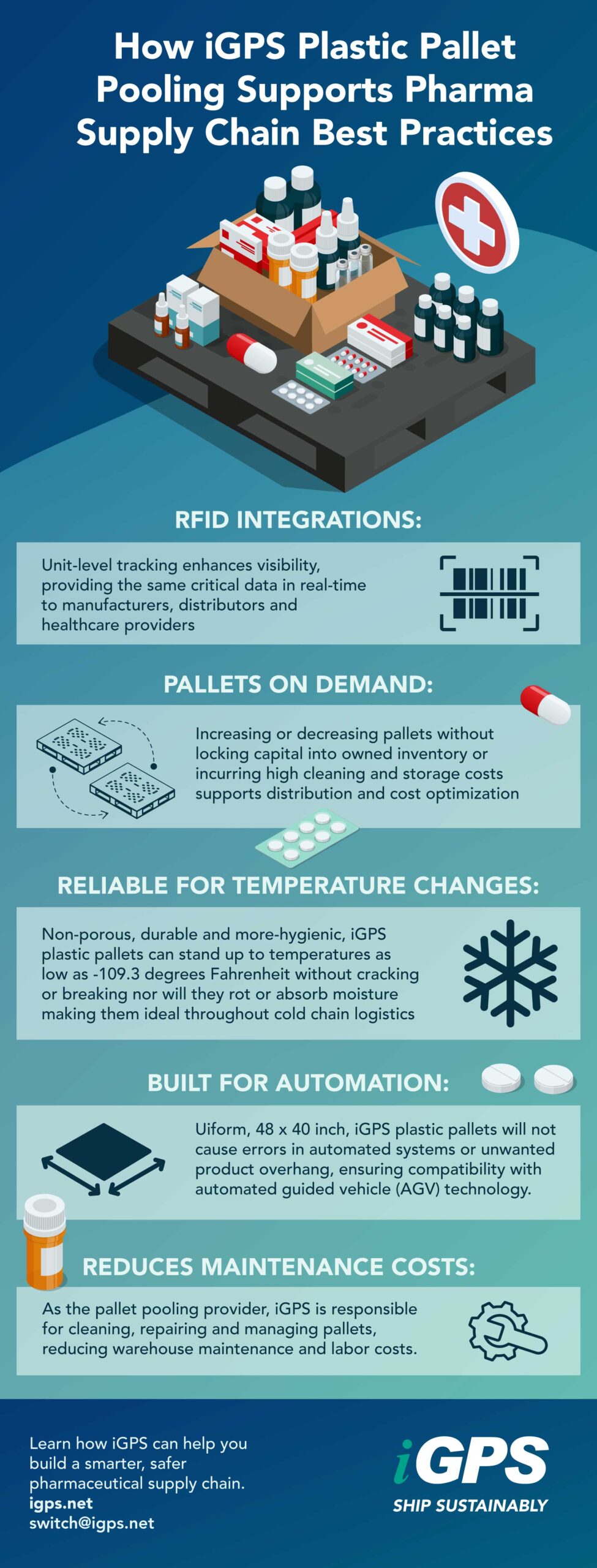
5 Pharma Supply Chain Best Practices
The future of pharmaceutical logistics depends on traceability, automation, and smart resource management.
1. Leverage Cloud-Based and Digital Platforms
Real-time data visibility is the cornerstone of modern supply chain strategies. Cloud-based platforms connect manufacturers, distributors, and PBMs, ensuring everyone has access to the same live data. This connectivity allows teams to track inventory, monitor temperature, and maintain compliance documentation seamlessly.
AI and predictive analytics also help forecast supply chain risks and prevent disruptions. RFID-integrated pallets, like those from iGPS, enhance visibility by providing unit-level tracking throughout the pharmaceutical supply chain.
2. Use Data to Forecast Demand and Optimize Distribution
Supply and demand fluctuations, or the “bullwhip effect,” can lead to costly inefficiencies. By analyzing usage patterns, patient trends, and seasonality, pharma companies can forecast demand accurately and design smarter distribution networks.
Plastic pallet pooling networks provide scalability, allowing pharmaceutical manufacturers to increase or decrease pallet availability on demand—without locking capital into owned inventory or incurring high cleaning and storage costs.
3. Maintain Temperature and Quality Control
Temperature control is vital to product efficacy and regulatory compliance. A single excursion can compromise drug integrity and increase the cost of production. Plastic pallets are ideal for cold chain logistics—they are non-porous, hygienic, and thermally stable, supporting airflow and consistent storage conditions. This helps ensure that pharmaceutical products remain safe, effective, and compliant with global quality standards.
By maintaining cold chain integrity, pharmaceutical manufacturers can safeguard product performance and prevent costly waste or recalls.
4. Strengthen Security and Traceability
Counterfeit and diverted drugs are a persistent challenge for pharmaceutical manufacturers and regulators. RFID and blockchain-based tracking systems create a digital chain of custody that ensures authenticity and protects patient safety.
With RFID-enabled pallet pooling, each shipment becomes traceable from source to point of sale, preventing counterfeit infiltration and ensuring adherence to compliance standards.
5. Continuously Audit and Optimize Processes
Continuous improvement is essential for resilient supply chains. Regular audits, supplier scorecards, and automated reporting help companies identify inefficiencies, reduce costs, and stay compliant with changing regulations.
What are Pharmaceutical Supply Chain Challenges
Standardization, technology, and pallet pooling can help reduce the impact of common pharmaceutical supply chain challenges:
- Limited Visibility and Data Fragmentation: Disparate systems and manual tracking make it difficult to trace materials or detect disruptions in real time.
RFID-enabled pallet pooling networks help improve shipment visibility and traceability by creating a consistent, data-connected foundation for unit-level tracking. - Supplier Concentration and Global Dependency: Relying heavily on specific regions or suppliers increases vulnerability to shortages or delays.
Expanding geographic diversification and leveraging domestic manufacturing capacity improves redundancy and reduces risk during global disruptions. - Regulatory Diversity Across Markets: Differing global standards for handling, documentation, and hygiene increase compliance costs.
Using standardized, hygienic plastic pallets within a managed pooling system helps maintain consistent quality and traceability documentation across borders, supporting regulatory compliance. - Rising Operational and Labor Costs: Owning, cleaning, and managing pallets in-house adds labor and maintenance overhead.
Partnering with a pooling provider shifts these responsibilities to a managed network, reducing warehouse maintenance costs and freeing resources for innovation and quality initiatives. - Cybersecurity and Digital Risk: As visibility systems go digital, data protection becomes essential.
Secure, cloud-based traceability platforms integrated with RFID-enabled assets can protect shipment data while ensuring transparency throughout the supply chain.
iGPS plastic pallets, for example, incorporate smart features like standardized identifying numbers that not only make them readable by all types of automated systems, but traceable at any point in the supply chain.
Typical Pharmaceutical Supply Chain Flow
A modern pharmaceutical supply chain combines physical logistics with digital intelligence:
- Sourcing: Verified suppliers provide APIs and raw materials under strict GMP oversight.
- Manufacturing: Drug products are formulated, tested, and packaged using standardized, quality-controlled processes.
- Packaging & Quality Testing: Finished medicines are serialized, labeled, and validated for purity, stability, and accuracy.
- Distribution: Wholesale distributors and logistics partners coordinate transportation, warehousing, and temperature control to prevent degradation.
- Storage & Delivery: Cold chain systems, smart sensors, and automated pallet cleaning maintain product hygiene and compliance during transit and reuse cycles.
- Point of Care: Pharmacies, hospitals, and healthcare providers dispense verified, traceable medications with complete digital documentation for every shipment.
Conclusion
The future of the pharma industry lies in creating digitally connected, physically sustainable supply chains. By combining advanced manufacturing technologies, AI-driven analytics, and RFID-enabled pooling systems, pharmaceutical manufacturers can enhance visibility, reduce risks, and deliver effective medications with confidence.
When your pallets are as intelligent and trackable as your products, your supply chain doesn’t just deliver medicines—it delivers trust.
FAQs
How Do Plastic Pallets Support the Pharma Supply Chain?
Plastic pallets play a vital role in maintaining clean, compliant, and efficient pharmaceutical logistics. Unlike wood pallets, they are non-porous, consistent in size, and easy to sanitize—making them ideal for GMP and GDP environments. Their uniformity supports robotic handling, RFID tracking, and automation, which enhance efficiency and reduce product damage.
By preventing moisture absorption, debris, and bacterial growth, plastic pallets help protect drug integrity and maintain quality standards from production to point of sale.
Why Is Renting or Pooling Plastic Pallets a Smarter Choice for Today’s Pharmaceutical Supply Chain?
Renting or pooling plastic pallets offers pharmaceutical manufacturers a cleaner, more cost-effective, and sustainable approach to logistics. Through iGPS’s nationwide pooling network, every pallet is AI-inspected, sanitized, and RFID-verified before reuse—eliminating contamination risk while reducing maintenance and replacement costs.
This on-demand, closed-loop model supports domestic manufacturing scalability, reduces waste, and strengthens the efficiency of global supply chains. It also helps companies meet sustainability and ESG goals while maintaining regulatory compliance and supply continuity.
How does iGPS improve visibility and traceability across the pharmaceutical supply chain?
Every iGPS pallet is RFID-enabled, providing real-time tracking from production to point of sale. This digital transparency supports compliance, helps prevent counterfeit infiltration, and enhances overall supply chain security.
How does iGPS support regulatory compliance for pharmaceutical manufacturers?
iGPS pallets meet strict GMP and GDP standards. Each pallet is inspected, sanitized, and verified for reuse—ensuring clean, traceable, and compliant handling of pharmaceutical products throughout the supply chain.
Pharmaceutical manufacturers across the U.S. are turning to iGPS plastic pallet pooling to improve compliance, traceability, and cost efficiency. Each RFID-enabled pallet is hygienic, durable, and verified for reuse—helping ensure product safety and GMP performance across every shipment. Contact 1-800-884-0225, email switch@igps.net, or visit the iGPS Contact Page to learn how iGPS can help you build a smarter, safer pharmaceutical supply chain.



