ARTICLE UPDATED ON FEBRUARY 28, 2024
By the conclusion of 2029, projections suggest that the pharmaceutical sector will expand to exceed $2.4 trillion in value. This substantial growth generates a substantial demand for the transportation of ethical drugs, requiring supply chain managers in the industry to be well-prepared. The fundamental pillars of secure pharmaceutical transportation encompass elements of basic importance: maintaining proper hygiene, ensuring security, monitoring temperature conditions, and meticulous record-keeping.
Nonetheless, the pharmaceutical industry comprises numerous intricate components, rendering the management of these critical factors a complex task. Fortunately, a handful of key tools can aid supply chain managers in effectively overseeing the pivotal aspects of pharmaceutical transportation. Employing radio frequency identification (RFID) tags for precise tracking and opting for plastic shipping pallets are two effective strategies to ensure that a supply chain remains adaptable to the evolving pharmaceutical shipping regulations and fluctuations in consumer demand.
The Four Cornerstones of the FDA’s Pharmaceutical Shipping Regulations

Pharmaceutical shipping regulations are covered extensively by the Food and Drug Administration (FDA) and apply to anyone who transports, stores, manufactures, or distributes wholesale drugs and the compounds to make them. Title 21, the code that deals with these regulations, is complex and lengthy but can be simplified into four primary areas of focus:
- Hygiene: Facilities that handle and store pharmaceutical products must be large enough to ensure proper cleaning, storage, and airflow around pallets housing products. There should also be a separate quarantine area for any medications which are damaged, outdated, or deteriorating. Any signs of infestation from animals, insects, or mold will cause a shipment to become unusable, so proper warehouse pest control and mold prevention practices, whether in the warehouse or the trailer, are essential.
- Security: Entry into a space where medications are being stored or transported must be carefully controlled and available only to authorized personnel. Surveillance cameras, proper lighting, access control keys, and automatic locking doors are all vital components of a successful security plan.
- Temperature: Protecting the integrity of the cold chain is arguably one of the most important parts of pharmaceutical supply chain management. Typically, ethical pharmaceuticals require storage between 59- and 77-degrees Fahrenheit. A fluctuation of only a few degrees could damage the efficacy of a product. If there are no specific storage temperatures listed for a product, the FDA requires them to be held at a “controlled” room temperature, which means that the temperature is that of a customary working environment and does not fluctuate drastically. This requirement must also be followed during transportation.
- Examination and recordkeeping: Inspections are a near-constant when shipping pharmaceuticals. Whenever a shipping container with pharmaceuticals is received, a thorough inspection should be performed for any signs of contamination. Managers who encounter such signs – for example, broken pallets, torn stretch wrap, or an obvious pest infestation – are required to refuse the shipment. All examinations must be logged either on paper or into a warehouse management system (WMS).
Paying close attention to these four broad categories of pharmaceutical shipping regulations can enormously reduce the risk of rejected loads and expensive wasted products. To simplify the tasks involved in following transportation guidelines, supply chain managers often choose to switch to easily trackable plastic pallets.
Simplifying Regulatory Compliance in the Pharma Supply Chain
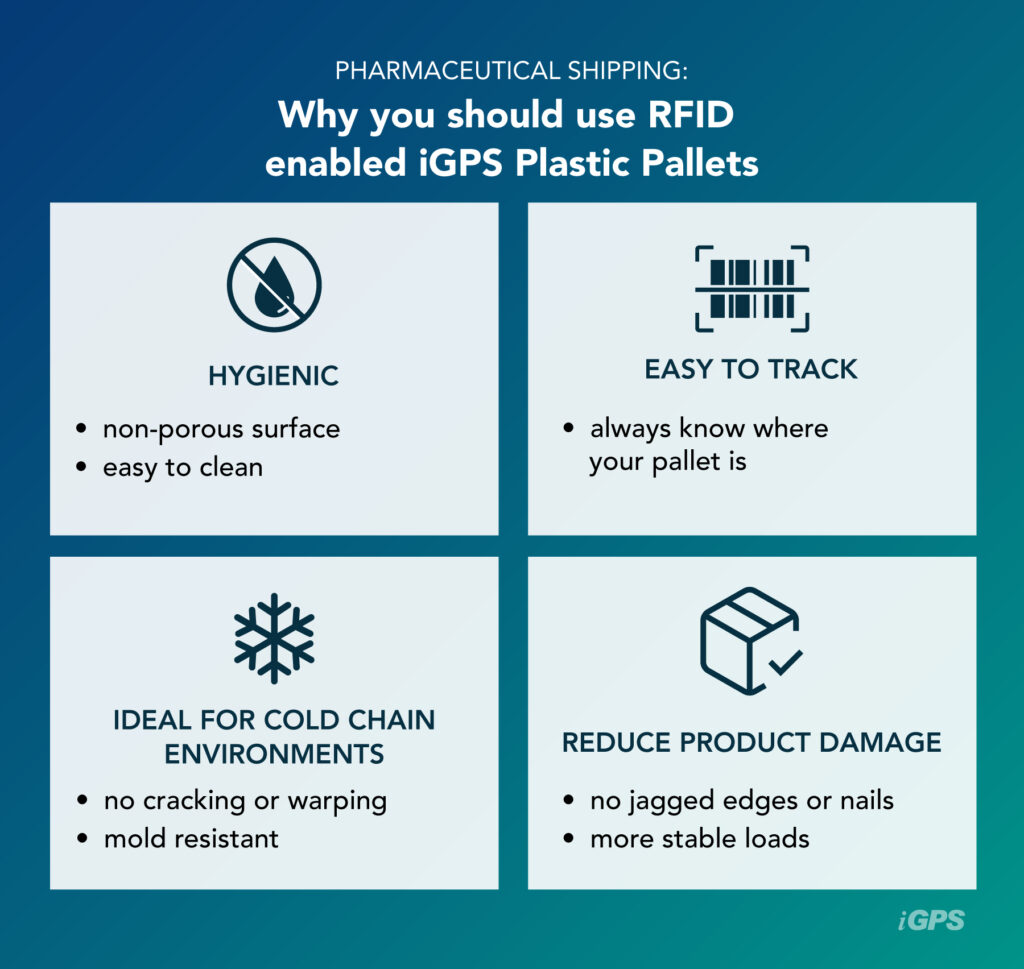
RFID-enabled plastic pallets can help businesses in the pharmaceutical industry meet requirements and ensure safety and compliance with pharmaceutical shipping regulations. Plastic shipping platforms simplify many common tasks by providing the following advantages:
- Hygienic: Because of its non-porous surface, plastic is very easy to clean. Spills, dirt, and debris won’t sink deep into the pallet and become difficult to remove like they would a traditional wooden pallet. Plastic pallets are also less likely to grow mold, as plastic dries thoroughly and doesn’t hold moisture.
- Easy to track: Embedded RFID tags make it easier to scan pallets and keep track of where they’ve been and where they are going. While it is possible to put RFID tracking tags on wood block pallets, it is less common as these platforms frequently break or lose boards. Instead, wooden pallets are typically tracked using less sophisticated barcodes.
- Ideal for cold chain environments: Supply chains with highly regulated temperatures can wreak havoc on wood pallets, as the condensation that collects on pallets as they’re moved between environments can seep into the pores of the wood and may even freeze, cracking or warping the pallet and potentially causing pallet failure. Because of condensation, pallets in cold chain environments also face increased issues with mold, making non-porous and easy-to-clean plastic an ideal material for cold chain pallets.
- Reduce product damage: Plastic pallets have no jagged edges or nails that could puncture packaging and lead to expensive load rejection. They are also sturdy and uniform in dimensions, ensuring stability in loads that ultimately reduces product damage during shipment and handling.
Compliance with pharmaceutical shipping regulations leaves no margin for error. Failing to adhere to these stringent guidelines can lead to supply chain managers facing substantial financial losses, potentially in the millions, and can compromise consumer safety. Incorporating plastic pallets as a fundamental component of your pharmaceutical shipping strategy helps with mitigating these potential risks.
Companies committed to following pharmaceutical shipping regulations should consider iGPS plastic pallets for all their shipping needs. Our lightweight, recyclable plastic pallets incorporate RFID technology, making them traceable throughout a supply chain. For more information, contact us at 1-800-884-0225, email a specialist at [email protected], or visit our contact page.




